1- Introduction 2- Cellular level 3- Development 4- Organ system involve
5- function 6- Mechanism 7- Related testing 8- Pathophysiology
9- Clinical significance
(1)- Introduction --
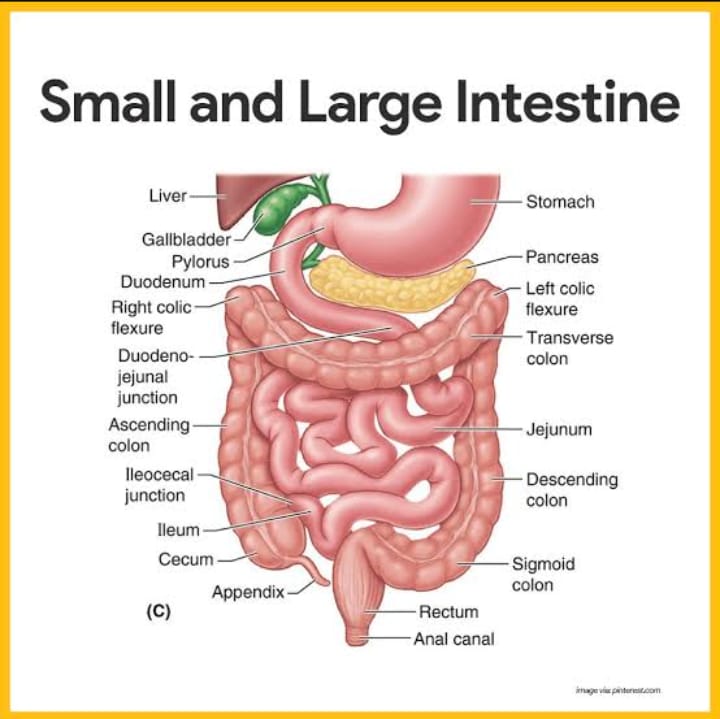
The small intestine (small bowel) is a hollow, tubular structure with an average adult length of 22 feet (7 meters), making it the longest portion of the gastrointestinal (GI) tract and where the majority of digestion occurs. The small intestine extends from the stomach pylorus to the ileocecal junction and is subdivided into three sections: the duodenum, jejunum, and ileum. It takes up to five hours for a single meal to be processed through the entire length of the small intestine, during which time it coordinates with the stomach, gallbladder, and pancreas to cue digestive juices to break down and absorb 95% of food nutrients. The small intestine extracts excess water and sends the remaining food waste to the large intestine to be formed into a stool.
The small intestine is found coiled inside the lower portion of the abdominal cavity, sitting beneath the stomach, and framed by the large intestine. When empty and at rest, the width of the small intestine is about the width of an index finger. This small width gives it the name of the small intestine, not its length. The large intestine is shorter but wider.
(2)- Cellular Level --
The small intestine is comprised of four layers, from innermost to outermost: mucosa, submucosa, muscularis propria, and serosa.
(1)- Mucosa: The mucosa is the innermost layer of the small intestine, lined by simple columnar epithelium, and consists of absorptive cells (enterocytes), goblet cells, and enteroendocrine cells. Enterocytes have approximately 3000 microvilli on their surfaces, helping to increase the surface area to facilitate effective absorption. Evaginations termed plicae circularis and villi are found in the mucosal layer and work to increase the surface area for maximal absorption. Invaginations, or crypts, are also found in the mucosal layer and are the protected site for stem cells. It is within the crypts that cell renewal and proliferation take place.
(2)- Submucosa: A layer of connective tissue containing blood vessels, nerves, and lymphatics. This layer of the small intestine is primarily unspecialized, except in the duodenum, where it contains mucous-secreting Brunner's glands.
(3)- Muscularis propria: The muscularis propria is made up of two layers of smooth muscle: a thin, outer longitudinal layer and a thick, inner circular layer. The outer longitudinal layer is responsible for shortening and elongating the intestine, while the inner circular layer is responsible for contraction. Between these two layers of smooth muscle is the myenteric (Auerbach) plexus of the enteric nervous system (ENS), which serves as the neuronal regulator of intestinal movement.
(4)- Serosa: The outermost layer of the small intestine consisting of epithelium and mesothelium. The serosa covers the jejunum, ileum, and the anterior surface of the duodenum. The posterior aspect of the duodenum is retroperitoneal and not covered by serosa.
(3)- Development --
Development of the small intestine begins at week three of gestation and is comprised of three stages: (1) morphogenesis and cell proliferation, (2) cell differentiation, and (3) functional maturation. Gastrulation, or the process of primitive gut tube formation, generates three primary germ layers: ectoderm, mesoderm, and endoderm. Multiple genes facilitate the formation of the small intestine, including SOX9, GATA4, and FOXA2. The Hox signaling pathway, involving the interplay between the endoderm and mesoderm, determines the different features of the small intestine.
Around week four of gestation, the premature midgut is open to the yolk sac, which grows more slowly than the developing embryo. Eventually, the edges of the embryonic disc come together with the midgut, forming the lumen of the small intestine, while the yolk sac becomes the vitelline duct.
By week five, the midgut has grown to the point of folding onto itself, while week six is when the loops of the gut herniate through the umbilicus, allowing for more room for the developing bowel, liver, and kidneys to grow. Weeks nine to 10 is when the small bowel returns to the abdomen.
During week six herniation, a 90-degree counterclockwise rotation occurs, which causes the ileum to reach the right upper quadrant of the abdomen. When the bowels return to the abdomen, another counterclockwise rotation of 180 degrees occurs, causing the cecum to now sit in the right upper quadrant.
Around week 11, the villi and microvilli form, and the small intestinal components are in their final arrangement. Throughout early development, the epithelium of the small intestine goes through recurrent turnover via stem cells. Originating from neural crest cells, the ENS is completed by week 13 of development.
The development of the small bowel is regulated by growth and transcription factors, such as epidermal growth factors (EGF), insulin-like growth factor (IGF), transforming growth factor-beta (TGF-beta), and hepatocyte growth factor (HGF). Late in gestation, fetal cortisone levels in the blood rise and aid in the process of gut development.
While most of the small intestine is part of the midgut, the duodenum is both a part of the foregut (anterior gut) and the midgut. The first (superior) part of the duodenum is a part of the foregut. The second (descending) part of the duodenum proximal to the ampulla of Vater is a part of the foregut, while the second (descending) part of the duodenum distal to the ampulla of Vater is a part of the midgut. The third (horizontal) and fourth (ascending) parts of the duodenum are a part of the midgut. The first part of the duodenum, as well as the jejunum and ileum, are intraperitoneal organs, while the 2nd-4th parts of the duodenum are retroperitoneal.
(4)- Organ Systems Involved --
To be the site of major absorption and digestion of nutrients, the small intestine must rely on enzymes supplied by multiple organs, including the stomach, gallbladder, pancreas, and salivary glands. Due to the involvement of these organs, malabsorption issues and clinical diagnoses may stem from regions other than the small intestine. For example, pancreatic insufficiency, pernicious anemia, and cystic fibrosis are all diseases involving malabsorption, yet they do not originate in the small intestine.
(5)- Function --
The primary function of the small intestine is to break down food, absorb nutrients, extract water, and move food along the gastrointestinal tract. The specific functions of each part of the small intestine are detailed below.
(1)- Duodenum - chemical digestion -
The pylorus of the stomach connects to the first part of the small intestine, the duodenum, which receives food from the stomach through the pylorus. Descending and short (10 inches long), the duodenum curves around the pancreas in a "C" shape and receives digestive juices from the liver, pancreas, and gallbladder via ducts connecting these organs to the duodenum. In the presence of food, hormone glands in the lining of the duodenum signal these organs to release their chemicals.
The duodenum is innervated by the celiac plexus, with the sympathetic innervation being the greater and lesser splanchnic nerves and the parasympathetic innervation being the vagus nerve.
Anatomically, the duodenum begins at the duodenal bulb and ends at the Ligament of Treitz, often referred to as the duodenojejunal (DJ) flexure. The duodenum is the site of iron and some folate absorption.
The duodenum is divided into four distinct parts:
1- Superior
- Most common site of duodenal ulcers
- A posterior duodenal ulcer in the superior segment of the duodenum may damage the gastroduodenal artery.
2- Descending
- Contains the ampulla of Vater and sphincter of Oddi
3- Horizontal
- The portion of the duodenum where superior mesenteric artery (SMA) syndrome occurs
- Anteriorly: superior mesenteric vessels
- Posteriorly aorta
4- Ascending
(2)- Jejunum - mechanical digestion -
The second part of the small intestine, the jejunum, is located mainly in the left upper quadrant (LUQ) of the abdomen and is the site of folate (B9) absorption. The jejunum has many blood vessels, giving it a deep red color on examination. The myenteric plexus triggers the intestinal walls of the jejunum to undergo segmentation, churning food back and forth and mixing it with digestive juices. Peristalsis, the moving of food down the digestive tract, also occurs here.
The mucosa of the duodenum and jejunum is thick with many folds and projections, making its surface area 100x greater than that of the skin. This physiology allows 95% of carbohydrates and proteins to be absorbed in the small intestine, as well as 90% of the water it receives during digestion, with the rest being absorbed in the large intestine.
(3)- Ileum -
The ileum is the third and final part of the small intestine. Located mainly in the right lower quadrant (RLQ) of the abdomen, the ileum is the longest section of the small intestine. The ileum is thinner, with more narrow walls and fewer blood vessels. Food spends most of the time in the ileum. Within the ileum, segmentation slows, and peristalsis takes over. The ileocecal valve separates the ileum from the large intestine (colon). Hormones and nerves signal the valve to open and close, allowing food to pass while keeping bacteria out. Special immune cells, Paneth cells, line the ileum to protect against bacteria. The ileum is the site of vitamin B12 and bile salt absorption.
Both the jejunum and ileum are innervated by the superior mesenteric plexus, a continuation of the lower portion of the celiac plexus, with the sympathetic innervation being the greater and lesser splanchnic nerves, while the parasympathetic innervation is the vagus nerve.
(6)- Mechanism --
Carbohydrates, proteins, and lipids are digested and absorbed by the small intestine. Dietary carbohydrates begin as polysaccharides, such as amylose, or disaccharides, such as lactose, sucrose, maltose, or trehalose. Each disaccharide is made up of two monosaccharides, which include glucose, fructose, and galactose. For the small bowel enterocytes to absorb carbohydrates, they must be broken down into monosaccharides. The enzyme responsible for carbohydrate digestion is amylase, which is found in the saliva and pancreas. Alpha-amylase is located in the mouth and begins the digestion of carbohydrates, while pancreatic amylase is primarily responsible for hydrolyzing starches. Along with amylase, the small intestine brush border contains enzymes that digest carbohydrates, including maltase, isomaltase, sucrase, beta-galactosidase, and trehalase complex. The monosaccharides are absorbed on the apical membrane via carrier-mediated transporters SGLT1 and GLUT5. Glucose and galactose use SGLT1 (fueled by sodium), while GLUT5 transports fructose.
Protein digestion occurs by breaking peptide bonds through hydrolysis by proteolytic enzymes. Proteins are broken down into their amino acids. Pepsin is a proteolytic enzyme in the stomach, where protein digestion starts. Chief cells secrete pepsinogen, an inactive precursor of pepsin, which can activate itself. Although pepsin helps digestion in the stomach, it is not completely necessary for protein digestion and must have an environment with a pH of 1 to 3 to work appropriately. Pepsin is not found in the small intestine, as the pancreatic fluids cause the duodenum to be a more basic environment. The pancreas secretes trypsin, chymotrypsin, elastase, and carboxypeptidase A and B. These proteases from the pancreas are released once the duodenum releases cholecystokinin (CCK) in the presence of proteins. The inactive forms of these pancreatic proteases are released first, and enteropeptidase (also called enterokinase) turns them into active forms.
Once trypsin is activated, it can auto-activate more of itself to hasten digestion. Protein digestion yields free amino acids, dipeptides, tripeptides, and oligopeptides. Dipeptides and tripeptides are transported via Pept-1, which requires a hydrogen ion gradient. Amino acid absorption involves many carrier-mediated active and facilitated transport proteins.
Dietary fats come in the form of triglycerides and are acquired primarily from animal sources. Triglycerides are broken down into 2-monoglycerides and fatty acids. Lipid digestion enzymes come from many areas prior to the duodenum, including lingual, gastric, and pancreatic lipases. CCK release from the duodenum causes sluggish gastric emptying, allowing more time for lipid digestion by the lingual and gastric lipases. There are also lipases within foods, termed food-bearing lipases, that can begin auto-digesting themselves. Colipase, lipase, phospholipase A2, and cholesterol ester hydrolase are pancreatic enzymes that break down fats. Bile salts cause the inactivation of lipases.
Colipase prevents lipase inactivation. Emulsification is essential in fat digestion as it breaks fat globules into smaller droplets, increasing the surface area for pancreatic lipases. Absorption of lipids was initially thought to be a passive process, yet the discovery of fatty acid-binding proteins supports an active course. Fatty acids are relocated to the endoplasmic reticulum of enterocytes and reformed. Free fatty acids are most commonly absorbed in the jejunum of the small intestine.
The small intestine also absorbs vitamins and minerals. Folate goes through hydrolysis, is absorbed in the duodenum and upper part of the jejunum, and is actively transported into the portal circulation. Vitamin B12 (cobalamin) is absorbed in the terminal ileum. B12 must bind to the R protein in the stomach. Once the B12-R complex reaches the duodenum, the R protein is hydrolyzed, and B12 binds to intrinsic factor (IF), secreted by the gastric parietal cells. The B12-IF complex travels to the terminal ileum and enters the enterocyte via ileal receptors. Vitamins A, D, E, and K are fat-soluble and may be passively absorbed in the small bowel. Approximately 9 liters of water travels to the gastrointestinal tract per day, and the small intestine absorbs 7 to 8 liters, while the colon absorbs the remaining 1 to 2 liters. Water absorption is thought to occur via osmotic gradients and aquaporins on intestinal membranes.
(7)- Related Testing --
Although thorough history-taking can uncover the cause of malabsorption in many cases, classic symptoms may not be present, and diseases can mimic each other. Diagnostic testing can include imaging studies, serology, and stimulation tests; however, workup will be driven by the clinical presentation.
Often, testing begins with routine labs when working up malabsorption. Labs typically include a complete blood count (CBC), comprehensive metabolic panel (CMP), iron studies, urinalysis, and coagulation panel. These blood tests can help support or discard specific diagnoses. Further diagnostic testing for suspected problems with absorption should be utilized based on individual patient presentation. These tests may include a combination of the following: qualitative and quantitative stool fat assessment, abdominal ultrasound, serologic markers for specific diseases such as celiac and Crohn disease, and colonoscopy, upper endoscopy, or sigmoidoscopy when warranted. Additional imaging of the small intestine with barium studies or magnetic resonance cholangiopancreatography (MRCP) and endoscopic retrograde cholangiopancreatography (ERCP) for suspected pancreatic etiologies of malabsorption may be necessary in individual cases.
When considering carbohydrate malabsorption, tests such as the lactose tolerance test and D-xylose test (measuring the capacity of small bowel absorption and aiding in mucosal etiologies) may be used.[20] Direct stimulation tests may be used to detect pancreatic insufficiency, along with measuring fecal elastase-1. Lastly, the Schilling test is an extensive method to identify a B12 deficiency, yet it is rarely necessary for the clinical setting.
(8)- Pathophysiology --
As the small bowel is the longest section of the GI tract, responsible for significant absorption and digestion, one can infer that numerous conditions can arise should the small intestine not function properly. Broadly, these can include congenital deformities, growths and obstructions, chronic diseases, and infections. A non-exhaustive list below, with brief explanations, highlights a few important small bowel pathologies.
(1)- Small bowel obstruction (SBO) -
Mechanical blockage of the small bowel with the greatest incidence occurring due to post-surgical adhesions: The second most common etiology of an SBO is a strangulated hernia, followed by malignancy, inflammatory bowel disease, foreign bodies, volvulus, and stool impaction.
(2)- Enteritis -
Inflammation of the small intestine, most commonly caused by radiation exposure or viral/bacterial pathogens (e.g., Norovirus, C. diff, E. Coli)
When the stomach is also affected, it is termed gastroenteritis. When the colon is also affected, it is termed enterocolitis.
(3)- Peptic ulcer disease (PUD) -
Erosion of the inner lining of the GI tract due to increased gastric acid secretion
Typically occurs in the stomach and proximal duodenum but may also involve the esophagus, distal duodenum, or jejunum.
(4)- Irritable bowel syndrome (IBS) -
Not to be mistaken for irritable bowel disease, IBS, also called spastic colon, is a poorly understood syndrome that results in abdominal pain, bloating, diarrhea, and constipation. IBS is characterized by motility disturbances and visceral hypersensitivity.
(5)- Intestinal pseudo-obstruction -
Signs and symptoms of bowel obstruction (e.g., dilated bowel with abdominal pain, nausea, and vomiting) without an identified anatomic obstruction.
(6)- Celiac disease -
Also called gluten-sensitive enteropathy, celiac disease is an autoimmune disease of the small intestine in which the body responds to gluten intake with an inappropriate immune response, leading to small intestine inflammation and damage.
(7)- Crohn disease -
An autoimmune disease characterized by full-thickness inflammation of the GI tract that may lead to fistulas and strictures. Approximately one-third of Crohn disease patients have involvement of the small bowel, most typically within the terminal ileum.
(8)- Meckel diverticulum -
Incomplete obliteration of the omphalomesenteric duct during embryological development of the GI tract leads to a diverticulum (outpouching) of the small intestine.
Typically remembered by the 'rule of 2's'. It occurs in 2% of the population, typically in children under two years of age, usually located two feet proximal to the ileocecal valve, affects males 2x as often as females, and is two inches long or less.
(9)- Short bowel syndrome (SBS) -
SBS may occur as a genetic defect at birth or following surgical resection of the small bowel.
As the small bowel is the site of most nutrient and water absorption, SBS is characterized by an inability to absorb fluids and vital nutrients.
(10)- Small intestinal bacterial overgrowth (SIBO) -
SIBO is characterized by an overall increase in bacteria of the small intestine and typically occurs following surgery, when the passage of food and waste slows through the GI tract, leading to optimal conditions for bacterial overgrowth.
(11)- Superior mesenteric artery (SMA) syndrome -
As previously mentioned, the third (horizontal) part of the duodenum sits between the superior mesenteric artery and the aorta.
SMA syndrome typically manifests in patients who have undergone substantial weight loss, as the mesenteric fat pad between the duodenum and the vasculature structures becomes smaller, and the duodenum is at an increased risk of compression by these structures.
(12)- Duodenal atresia -
A congenital defect caused by failure of recanalization of a portion of the duodenum, leading to complete occlusion or absence of the duodenal lumen.
Presents with vomiting in the first 24 to 36 hours of life. Surgical repair via duodenoduodenostomy is necessary.
(13)- Necrotizing enterocolitis -
Hemorrhagic necrotizing inflammation of the intestinal wall of premature neonates, most commonly occurring in the distal ileum and proximal colon. While the causes are not entirely understood, necrotizing enterocolitis is thought to occur due to an underdeveloped immune system.
(14)- Intussusception -
It occurs when the proximal portion of the small bowel telescopes into the distal portion, leading to obstruction and bowel ischemia.
(15)- Enterocele (small bowel prolapse) -
A type of pelvic organ prolapse in which a portion of the small bowel descends into the pelvic cavity, creating a bulge in the vagina. Those most likely to develop an enterocele include multiparous and post-menopausal women.
(16)- Carcinoma of the small intestine -
Small bowel cancers comprise < 5>
(9)- Clinical Significance --
As a clinician, it is essential to counsel patients on the importance of maintaining proper gut health by moderating alcohol intake and tobacco use and intaking adequate amounts of fiber and water. In addition, patients should be educated on the importance of moderate NSAID use, as the two leading causes of ulcers in the stomach and duodenum are NSAIDs and H. Pylori. NSAIDs erode the stomach lining, leading to high stomach acid, which can leak into the duodenum and make it vulnerable to bacterial infections such as H. Pylori.
It is clinically important to understand the anatomy of the small bowel, as swallowed foreign bodies are most likely to become lodged in the most narrow parts of the intestine, which include the pylorus, the DJ flexure, and the ileocecal junction.
Malabsorption disorders can be classified as either global, involving the entire mucosa, or partial, dealing with the malabsorption of specific nutrients. Clinical features of malabsorption are relatively specific to the type of nutrient that is deficient, yet weight loss and fatty, greasy stools are classical findings of global malabsorption. Stools are typically voluminous and pale. Most patients present with nonspecific gastrointestinal symptoms such as abdominal pain, flatulence, anorexia, and distension. Patients with malabsorption issues may also be asymptomatic.
During instances of isolated malabsorption, such as celiac disease, specific manifestations, such as bone thinning or iron deficiency anemia, might be the only presentation. Malabsorption of carbohydrates can cause milk intolerance and watery diarrhea, while protein malabsorption can lead to muscle atrophy and menstrual irregularities. Additionally, folic acid malabsorption and iron deficiency lead to anemia, vitamin A deficiency may cause night blindness, and vitamin K deficiency can lead to bleeding disorders. It is also important to note that some causes of malabsorption, such as the malabsorption of fats from celiac disease or pancreatic insufficiency, can cause the malabsorption of other substances, such as vitamin D, which needs chylomicrons to be absorbed effectively.
Based on the clinical implications of malabsorption, it is crucial to recognize that these diseases can cause great hindrances in patients’ lives, altering their ability to go about their daily activities. It is an important topic to continue researching as more pediatric patients are reaching adulthood with diseases such as short bowel syndrome, cystic fibrosis, and inflammatory bowel disease.
2023 © DL NEWS. All Rights Reserved.